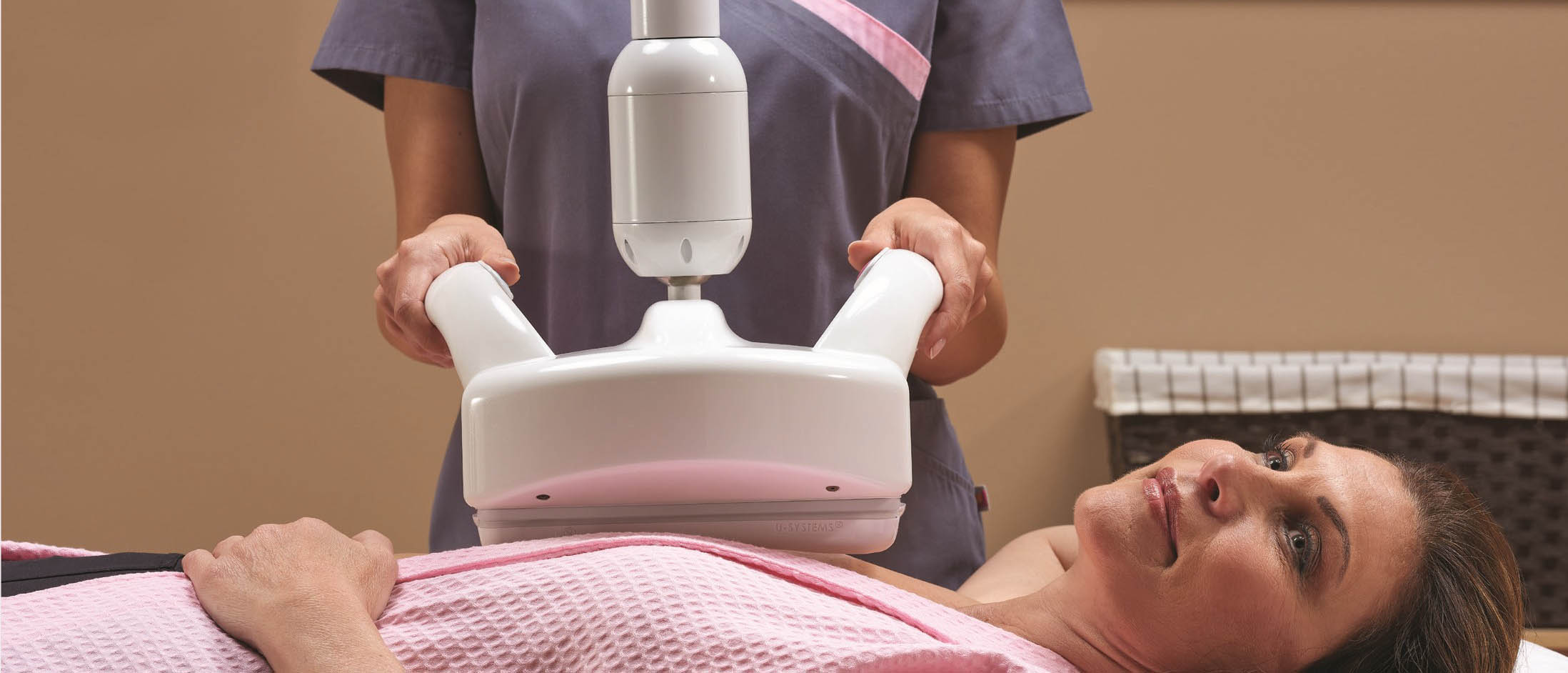
The Dense Breast Dilemma
Over 40 percent of women have dense breast tissue, one of the strongest common risk factors for developing breast cancer. In dense breasts, cancers may be masked on 2D mammography – potentially delaying diagnosis in these women. Both dense breast tissue and cancer appear white on a mammogram, creating a dangerous camouflage effect and a dilemma for radiologists whose goal is to find breast cancer as early as possible.
Detecting More Cancer
GE Healthcare offers the only FDA-approved ultrasound supplemental screening technology that is specifically designed for detecting cancer in dense breast tissue. Compared to mammography alone, Invenia ABUS 2.0 imaging looks differently at dense breast tissue, providing a comprehensive view of the breast. This technology has demonstrated a 35.7% increase in cancer detection over mammography alone for these women.
Supplemental imaging with Invenia ABUS 2.0 transforms breast care from reactive to proactive. Clinical research studies demonstrate that when used as an adjunct to mammography, small cancers visible only through ABUS were predominantly invasive and node-negative. Detecting them at this earlier stage has important prognostic implications and can reduce the cost of care.
Learn More about Abus 2.0
Contact Tower Saint John’s Imaging to learn more about Automated Breast Ultrasound technology and how it can help save lives.
